
����Ŀ����ϩ����Ҫ����ԭ�ϡ��������·�ش��������⡣
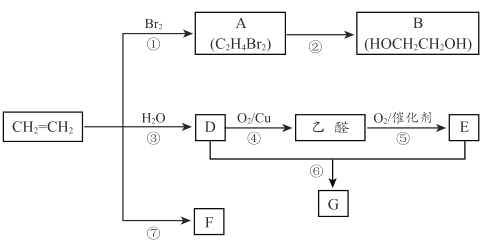
(1)��Ӧ�ٵĻ�ѧ����ʽ�� ____________________��
(2)B��������_______________��
(3)��Ӧ�ܵĻ�ѧ����ʽ��_________________��
(4)F��һ�ָ߷������ʣ�����������ʳƷ���ϴ��ȣ�F �Ľṹ��ʽ��__________��
(5)E�ķ���ʽ�� C2H4O2��ʹ��ɫʯ����Һ��죻 G ��һ����״������ζ�����ʣ�ʵ������ D �� E ͨ����Ӧ����ȡ G, װ����ͼ��ʾ��
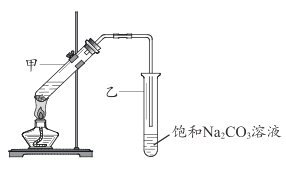
i.���Թ��з�Ӧ�Ļ�ѧ����ʽ�� __________����Ӧ������_________��
ii.������Թ�������״Һ���õ�����Ҫ������___________________��
iii.����� 4. 6g D �� 3g E �ڴ��������·���������Ӧ ����ַ�Ӧ�����ʵ�ʲ���Ϊ60%,ʵ�ʵõ�G ��������__________ g��(��֪��ʵ�ʲ��ʣ� ʵ�ʵõ����������ۼ�������)
(6)��ϩ( CH3CH = CH2 ) ����ϩ��Ϊͬϵ��ڴ�����������������O2 ��Ӧ����һ����Ҫ�Ļ���ԭ�ϱ�ϩ��( CH2= CHCOOH )�����й�����ϩ����˵����ȷ���� ________��
a.�����ụΪͬϵ��
b.�ܷ����ӳɡ�������������Ӧ
c.���� NaHCO3��Һ��Ӧ���� CO2
d.һ���������ܷ����Ӿ۷�Ӧ������![]()
���𰸡�CH2=CH2+Br2=BrCH2CH2Br �ǻ���-OH�� ![]()
![]()
![]() ������Ӧ����ȡ����Ӧ�� ��Һ©�� 2.64g bc
������Ӧ����ȡ����Ӧ�� ��Һ©�� 2.64g bc
��������
��ת������ͼ��֪��CH2=CH2���嵥�ʼӳ�����BrCH2CH2Br��BrCH2CH2Br��NaOHˮ��Һ�й��ȷ���ˮ�ⷴӦ������HOCH2CH2OH��CH2=CH2��ˮ�����Ҵ�CH3CH2OH���Ҵ�CH3CH2OH������Ϊ��ȩCH3CHO����ȩCH3CHO�ڴ�����Ϊ����CH3COOH��CH3COOH��CH3CH2OH��Ũ�����������������������CH3COOCH2CH3����ϩCH2=CH2�ڴ�����һ�������·����Ӿ۷�Ӧ���ɾ���ϩ���ݴ˷������
(1)��Ӧ������ϩ����ļӳɷ�Ӧ����1��2-�������飬�仯ѧ����ʽΪ��CH2=CH2+Br2=BrCH2CH2Br���ʴ�Ϊ��CH2=CH2+Br2=BrCH2CH2Br��
(2)B���Ҷ���������������ǻ���-OH�����ʴ�Ϊ���ǻ���-OH����
(3)��Ӧ��Ϊ�Ҵ��Ĵ�������Ӧ���仯ѧ����ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(4)F��һ�ָ߷������ʣ�����������ʳƷ���ϴ��ȣ�![]() F������ϩ������ṹ��ʽΪ��
F������ϩ������ṹ��ʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(5) i�����Թ������Ʊ����������ķ�Ӧ�����仯ѧ����ʽ��![]() ���÷�Ӧ����������Ӧ��Ҳ����ȡ����Ӧ���ʷ�Ӧ������������Ӧ��ȡ����Ӧ���ʴ�Ϊ��
���÷�Ӧ����������Ӧ��Ҳ����ȡ����Ӧ���ʷ�Ӧ������������Ӧ��ȡ����Ӧ���ʴ�Ϊ�� ![]() ��������Ӧ����ȡ����Ӧ����
��������Ӧ����ȡ����Ӧ����
ii���������ֻ������ݵ�Һ�壬���÷�Һ�ķ������з��룬��Ҫ�����Ƿ�Һ©�����ʷ�����Թ�������״Һ���õ�����Ҫ�����Ƿ�Һ©�����ʴ�Ϊ����Һ©����
iii�����ݷ���ʽ���м��㣬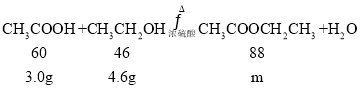 ��֪m=4.4g����ʵ�ʲ���Ϊ60%����ʵ�ʵõ���������������Ϊ4.4g��60%=2.64g���ʴ�Ϊ��2.64g��
��֪m=4.4g����ʵ�ʲ���Ϊ60%����ʵ�ʵõ���������������Ϊ4.4g��60%=2.64g���ʴ�Ϊ��2.64g��
(6)
a����ϩ���к����Ȼ���̼̼˫������������ֻ���Ȼ������߽ṹ�����ƣ��ʲ���Ϊͬϵ�a����
b����ϩ���к����Ȼ���̼̼˫���ȹ����ţ����ܷ����ӳɡ�������������Ӧ��b��ȷ��
c����ϩ���к����Ȼ���������NaHCO3��Һ��Ӧ����CO2��c��ȷ��
d��һ���������ܷ����Ӿ۷�Ӧ����������Ľṹ��ʽΪ��![]() ����d����
����d����
�ʴ�Ϊ��bc��


 ������ϵ�д�
������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ���ڼӳɷ�Ӧ����
A.��ϩ��������Ӧ��������B.�Ҵ����Ʒ�Ӧ�����Ҵ���
C.������������Ӧ����һ�ȼ���D.�������Ҵ���Ӧ������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ��������ͼ��ʾװ�����Ʊ���Ȳ������֤��Ȳ��ijЩ��ѧ���ʣ��Ʊ�����Ȳ��������������������H2S���壬�밴����Ҫ����գ�
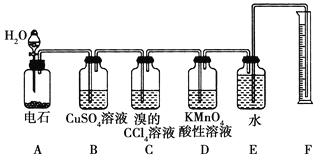
��1��ʵ��������Ȳ�Ļ�ѧ����ʽ��____________________________��Ϊ�˵õ���Ϊƽ�ȵ���Ȳ������װ��A�ķ�Һ©���г���________________������ˮ��
��2��װ��B��CuSO4��Һ��������________________��
��3��װ��C�й۲쵽��������_________________����Ӧ�Ļ�ѧ����ʽ��___________________��
��4��װ��D�й۲쵽��������_________________���÷�Ӧ��������______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ���ǣ� ��
A.CH3COOCH2CH3 ��CH3CH2COOCH3 �о����м����һ���������Ϊͬһ������
B.![]() ��
�� ![]() Ϊͬһ����
Ϊͬһ����
C.CH3CH2CH2CH2CH3 ��CH3CH2CH��CH3��2 ��Ϊͬ��������
D.CH3CH2OH �� CH2OHCHOHCH2OH ������ͬ�Ĺ����ţ���Ϊͬϵ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I��������CuSO4��ĩ����ʢ��ˮ���Թ��еõ�һ������ɫ��Һ�������Թ������Һ�еμӰ�ˮ�������γ���ɫ�����������μӰ�ˮ�������ܽ⣬�õ�����ɫ��Һ���ټ����Ҵ��ܼ�������������ɫ�ľ��塣
(1)��Һ�г�����ɫ���Ļ�ѧʽ��_______________________��
(2)�����Ҵ���������_____________________________��
(3)д����ɫ�����ܽ������ɫ��Һ�����ӷ���ʽ______________��
(4)�õ�������ɫ������[Cu(NH3)4]SO4��H2O��������Cu2+��NH3֮��Ļ�ѧ������Ϊ_____________�� �þ�����������ӵĿռ乹��Ϊ_______________________��(����������)
II����![]() ��FeԪ�ص���������������������Ҫ����;���ش��������⣺
��FeԪ�ص���������������������Ҫ����;���ش��������⣺
(1) ��K3[Fe(CN)6]������������________��������_________�������_________��
(2)ij��![]() (��)�л������Ľṹ��ͼ��ʾ��
(��)�л������Ľṹ��ͼ��ʾ��
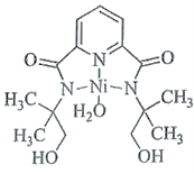
�ٸ÷�����Nԭ�ӵ��ӻ���ʽΪ________��________��
������ͼ������![]() �����
�����![]() �������_____
�������_____
(3) Ge��As��SeԪ�ش���ͬһ���ڣ�����Ԫ��ԭ�ӵĵ�һ�������ɴ�С��˳��Ϊ__________________��
(4)![]() ������ǿ��
������ǿ��![]() ��ԭ����_________________________________________��
��ԭ����_________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ������Fe��FeO��Fe2O3�Ļ�����м���60mL 4 mol��L��1��ϡ���ᣬǡ��ʹ�������ȫ�ܽ⣬�ų�1.344 L NO(��״��)����������Һ�м���KSCN��Һ����ɫ����.���������������ڼ����»�ԭ��ͬ�����Ļ����ܵõ��������ʵ���Ϊ
A.0.09 molB.0.12 molC.0.16 molD.0.21 mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
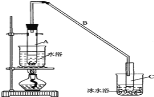
����Ŀ��ij��ѧС���������������������װ�ã���ͼ�����Ի������Ʊ�����ϩ��
�ܶȣ�g/cm3�� | �۵㣨�棩 | �е㣨�棩 | �ܽ��� | |
������ | 0.96 | 25 | 161 | ������ˮ |
����ϩ | 0.81 | ��103 | 83 | ������ˮ |
��֪�� +H2O
+H2O
��1���Ʊ���Ʒ����12.5mL�����������Թ�A�У��ټ���1mLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
��A�����Ƭ��������__������B���˵�������е�������__��
���Թ�C���ڱ�ˮԡ�е�Ŀ����__��
��2���Ʊ���Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ��__�㣨����������������������Һ����__�������ţ�ϴ�ӡ�
A.KMnO4��Һ B.ϡH2SO4 C.Na2CO3��Һ
�����ٽ�����ϩ����������ȴˮӦ��__�ڽ��루��������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ClO2��һ�����������������������Ƴ�NaClO2���壬�Ա���������棬�������ⷨ��NaClO2�����ʵ��װ����ͼ��ʾ��
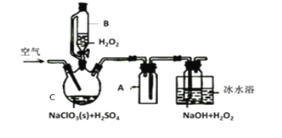
��֪����2NaC1O3+H2O2+H2SO4=2C1O2��+O2��+Na2SO4+2H2O
2ClO2+H2O2+2NaOH=2NaClO2+O2��+2H2O
��ClO2�۵�-59�桢�е�11�棬Ũ�ȹ���ʱ�����ֽ⣻
��H2O2�е�150��
��1����ˮԡ��ȴ��Ŀ����___��
��2���������ٹ���������������NaClO2���ʣ��Խ�����ԭ�������ٹ���ʱ��__��
��3��Cl-����ʱ���ClO2�����ɡ���Ӧ��ʼʱ��C�м����������ᣬClO2���������ʴ����ߣ����������������ù��̿��ܾ�������ɣ��뽫�䲹��������
��___�������ӷ���ʽ��ʾ����H2O2+Cl2=2Cl-+O2+2H+
��4��NaClO2���Ȳⶨ��
��ȷ��ȡ����NaClO2��Ʒ10.0g���ձ��У�������������ˮ�����ĵ⻯�ؾ��壬�ٵ���������ϡ���ᣬ��ַ�Ӧ��C1O2-�IJ���ΪCl-���������û��Һ���250mL������Һ��
��ȡ25.00mL����Һ����2.0mol��L-1Na2S2O3��Һ�ζ�(I2+2S2O32-=2I-+S4O62-)���Ե�����Һ��ָʾ�����ﵽ�ζ��յ�ʱ������Ϊ__���ظ��ζ�3�Σ����Na2S2O3��Һƽ������Ϊ20.00mL�������Ʒ��NaClO2����������Ϊ___����M(NaClO2)=90.5g/mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
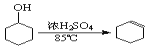
����Ŀ����֪��CH3CH2CH2CH2OH![]() CH3CH2CH2CHO��������ͼװ�����������ϳ�����ȩ������������
CH3CH2CH2CHO��������ͼװ�����������ϳ�����ȩ������������
���� | �е�/�� | �ܶ� / gcm-3 | ˮ���ܽ��� |
|
������ | 117.2 | 0.8109 | �� | |
����ȩ | 75.7 | 0.8017 | �� |
����˵���У�����ȷ����
A.Ϊ��ֹ�����һ��������Ӧ���ữ��Na2Cr2O7��Һ��μ�����������
B.���¶ȼ�1ʾ��Ϊ90~95�����¶ȼ�2ʾ����76������ʱ���ռ�����
C.��Ӧ������������ﵹ���Һ©���У���ȥˮ�㣬������ȩ�ӷ�Һ©���Ͽڵ���
D.���õĴ�����ȩ�м������������ƣ����������Ƿ���������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com