
| ���� | H2 | CO | CH4 |
| ȼ����kJ?mol-1 | 285.8 | 283.0 | 890.3 |
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
| X |
| 285.8+283.0 |
| 1 |
| 2 |
| 0.95X |
| 568.8 |
| 1 |
| 2 |
| X |
| 890.3 |
| ||
|



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
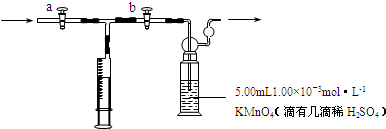
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������Ԫ����ԭ�Ӱ뾶��С����F |
B�����ݦм��ijɼ������ж�C-C�ļ�����  ���ܵĹ�ϵ��˫���ļ���С�ڵ����ļ��ܵ�2�� ���ܵĹ�ϵ��˫���ļ���С�ڵ����ļ��ܵ�2�� |
| C��Ԫ�ص縺��ԽС��Ԫ�طǽ�����Խǿ |
| D����n���ڵ�n�����Ԫ�ؾ�Ϊ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
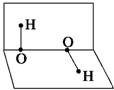 ��֪H2O2�ķ��ӿռ�ṹ���ڶ�����б�ʾ����ͼ��ʾ�����й�H2O2�Ľṹ��˵���в���ȷ���ǣ�������
��֪H2O2�ķ��ӿռ�ṹ���ڶ�����б�ʾ����ͼ��ʾ�����й�H2O2�Ľṹ��˵���в���ȷ���ǣ�������| A�����ӵ�������������IJ��غ� |
| B��H2O2�����ڼȺ����Լ��ֺ��Ǽ��Լ� |
| C��H2O2�Ǽ��Է��� |
| D��H2O2���Ӽ䲻�����γ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 A����;���Ľ�����B��C�����ֳ������嵥�ʣ�E��ҺΪ����ǿ�ᣬD��Һ�еμ�KSCN��Һ�Ժ�ɫ�������ת����ϵ��ͼ��ʾ��
A����;���Ľ�����B��C�����ֳ������嵥�ʣ�E��ҺΪ����ǿ�ᣬD��Һ�еμ�KSCN��Һ�Ժ�ɫ�������ת����ϵ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
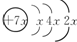 ��B��ͬ���ڳ�ϡ��������뾶����Ԫ�أ�C������������������ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
��B��ͬ���ڳ�ϡ��������뾶����Ԫ�أ�C������������������ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com