
����Ŀ��H2��CO��CH4��CH3OH�ȶ�����Ҫ����Դ��Ҳ����ҪΪ����ԭ�ϡ�
��1����֪25�棬1.01��105Paʱ��8.0g CH4��ȫȼ�����ɶ�����̼�����Һ̬ˮ�ų�444.8kJ������д���÷�Ӧ���Ȼ�ѧ��Ӧ����ʽ��___________________________________________��
��2��Ϊ���������ܼ��š��͡���̼���á������ʹ�����CO2�ĺ�������Ч�ؿ�������CO2����ҵ�Ͽ�����CO2������ȼ�ϼ״��������Ϊ2L���ܱ������У�����lmol CO2��3mol H2��һ�������·�����Ӧ��CO2(g) + 3H2(g) ![]() CH3OH(g) + H2O(g)�������CO2��CH3OH(g)�����ʵ�����ʱ��仯��ͼ��ʾ��
CH3OH(g) + H2O(g)�������CO2��CH3OH(g)�����ʵ�����ʱ��仯��ͼ��ʾ��
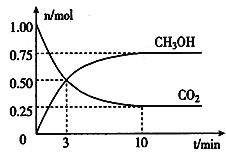
�ٴӷ�Ӧ��ʼ��ƽ�⣬CO2��ƽ����Ӧ����v(CO2)=___________________��
�ڴﵽƽ��ʱ��H2��ת����Ϊ__________��
�۸÷�Ӧ��ƽ�ⳣ��K=___________________������ʽ����
�����д�ʩ������߷�Ӧ���ʵ���__________��
A.�����¶� B.������� C.����ѹǿ D.��ʱ�����CH3OH
��3����ҵ��Ҳ��CO��H2Ϊԭ���Ʊ�CH3OH����Ӧ����ʽΪ��CO(g) + 2H2(g)![]() CH3OH(g)����һ����̶����ܱ�������Ͷ��һ������CO��H2�������������Ӧ��������������˵��������Ӧ�ﵽƽ��״̬����______��
CH3OH(g)����һ����̶����ܱ�������Ͷ��һ������CO��H2�������������Ӧ��������������˵��������Ӧ�ﵽƽ��״̬����______��
A.��Ӧ��CO��CH3OH�����ʵ���֮��Ϊ1:1
B.��������ѹǿ����ʱ��ı仯���仯
C.��λʱ����ÿ����1 mol CO��ͬʱ����1 mol CH3OH
D.CH3OH�����������ڻ�������б��ֲ���
E.���������ܶȱ��ֲ���
���𰸡� CH4(g) + 2O2(g) = CO2(g) + H2O(l) ��H=��889.6 kJ/mol 0.0375mol/(L��min) 75�� c(CH3OH)��c(H2O)/c(CO2)��c3(H2) D BD
����������1������n=m/M�����8.0g��������ʵ�����Ȼ��ɼ����1mol������ȫȼ������Һ̬ˮ�ų�����������������Ȼ�ѧ����ʽ����дԭ��д������ȼ�յ��Ȼ�ѧ����ʽ����2���ٸ�������v=��C/��t�������ʽ���м��㣻�ڴﵽƽ��ʱ��H2��ת����=��Ӧ�����������ʵ���/�����������ۻ�ѧ��Ӧ��ƽ�ⳣ��K=����������ƽ��Ũ��ϵ���η��ij˻�������Ӧ��ƽ��Ũ��ϵ���η��˻��ı�ֵ���ܸ�����������Է�Ӧ���ʵ�Ӱ����������������3����ѧƽ��״̬���������ȣ���V��=V�����������ﵽƽ���Ӧ����������ʵ�����������Ũ�ȣ��ٷֺ����ȱ��ֲ���ݴ��жϽ��
��1��8.0g CH4�����ʵ���Ϊ��8.0g/16g��mol��1=0.5mol��0.5molCH4��ȫȼ������Һ̬ˮ�ų�444.8kJ��������1mol CH4��ȫȼ������Һ̬ˮ�ų�������Ϊ��444.8kJ��1mol/0.5mol=889.6kJ�������ȼ�յ��Ȼ�ѧ����ʽΪ��CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6 kJ��mol��1����2�������Ϊ2L���ܱ������У�����lmol CO2��3mol H2��һ�������·�����Ӧ��CO2(g) + 3H2(g) ![]() CH3OH(g) + H2O(g)�������CO2��CH3OH(g)�����ʵ�����ʱ��仯��ͼ��ʾ��������̼�Ƿ�Ӧ���淴Ӧ�������ʵ�����С���״���������淴Ӧ�������ʵ�������10nim�ڴﵽƽ�⣬���ɼ״����ʵ���Ϊ0.75mol��������̼���ʵ����仯��0.75mol��������ݻ�ѧƽ����ʽ����Ϊ��
CH3OH(g) + H2O(g)�������CO2��CH3OH(g)�����ʵ�����ʱ��仯��ͼ��ʾ��������̼�Ƿ�Ӧ���淴Ӧ�������ʵ�����С���״���������淴Ӧ�������ʵ�������10nim�ڴﵽƽ�⣬���ɼ״����ʵ���Ϊ0.75mol��������̼���ʵ����仯��0.75mol��������ݻ�ѧƽ����ʽ����Ϊ��
CO2(g) + 3H2(g) ![]() CH3OH(g) + H2O(g)
CH3OH(g) + H2O(g)
��ʼ����mol�� 1 3 0 0
�仯����mol�� 0.75 2.25 0.75 0.75
ƽ������mol�� 0.25 0.75 0.75 0.75
CO2��ƽ����Ӧ����v��CO2��=0.75mol����2L����1��10min-1
=0.0375mol/(L��min)��
�ٴӷ�Ӧ��ʼ��ƽ�⣬CO2��ƽ����Ӧ����v(CO2)=0.0375mol/(L��min)���ڴﵽƽ��ʱ��H2��ת����Ϊ2.275mol/3mol��00%=75�����۸÷�Ӧ��ƽ�ⳣ��K=c(CH3OH)��c(H2O)/c(CO2)��c3(H2)������ʽ������A.�����¶ȼӿ췴Ӧ���ʣ���A����B.��������ӿ췴Ӧ���ʣ���B����C.����ѹǿ�ӿ췴Ӧ���ʣ���C����D.��ʱ�����CH3OH��Ũ�Ƚ��ͣ�������Ӧ���ʣ���D��ȷ����ѡD����3��A.��Ӧ��CO��CH3OH�����ʵ���֮��Ϊ1:1����ȷ���Ƿ�ﵽƽ��״̬��������Ϊ�ж�ƽ��ı�־��A����B.�÷�Ӧǰ�������������ȣ���ѹǿ����˵���ﵽƽ�⣬��B��ȷ��C.��λʱ����ÿ����1 mol CO�������ʣ�ͬʱ����1 mol CH3OHҲ�������ʣ���ָ�����ʶ��DZ�ʾ����Ӧ���ʣ�������Ϊ�ж�ƽ���־����C����D.CH3OH�����������ڻ�������б��ֲ��䣬CH3OH���һ����˵���ﵽƽ�⣬��D��ȷ��E.��Ӧ��Ͳ��ﶼ�����壬���������������䡢����������䣬���ܶ�ʼ�ղ��䣬��E����ѡBD��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼװ�ý���ʵ�飬��ʼʱ��a��b����Һ����ƽ���ܷ�ã�����һ��ʱ�䡣����˵������ȷ���� (����) 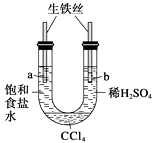
A.a�ܷ���������ʴ��b�ܷ������ⸯʴ
B.һ��ʱ���a��Һ�����b��Һ��
C.a����Һ��pH����b����Һ��pH��С
D.a��b����������ͬ�ĵ缫��Ӧʽ��Fe��2e���� Fe2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ˮ�Ǿ����Դ���⣬�Ӻ�ˮ����ȡʳ�κ���Ĺ������£�

��1����������ѻ��Br2����������ֽ�Br2��ԭΪBr-����Ŀ��Ϊ ___________ ��
��2���������SO2ˮ��Һ����Br2�������ʿɴ�![]() ���йط�Ӧ�����ӷ���ʽΪ_________________��
���йط�Ӧ�����ӷ���ʽΪ_________________��
��3���������Ͽ��ǣ���������Ҳ������Br2����____________��
A. H2O B. Na2CO3 C. Na2SO3 D. FeCl3
��4��ij��ѧ�о���ѧϰС��Ϊ�˽�ӹ�ҵ�����ᴿ��ķ������������й����ϣ�![]() �ķе�Ϊ
�ķе�Ϊ![]() ������ˮ���ж��Ժ�ǿ��ʴ�ԡ������IJ����ж�ʢ�й�ҵ���������ƿ��ȡ�ļ��ȷ�ʽ��__________ ��������������õ�Һ�����ˮ�Ļ������������ǵ�����ܶ����ϴ���ص���з��룬���õķ�������������__________ ��
������ˮ���ж��Ժ�ǿ��ʴ�ԡ������IJ����ж�ʢ�й�ҵ���������ƿ��ȡ�ļ��ȷ�ʽ��__________ ��������������õ�Һ�����ˮ�Ļ������������ǵ�����ܶ����ϴ���ص���з��룬���õķ�������������__________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ̽�������ĸ�ʴ�����ijͬѧ��пƬ��ͭƬ���ڽ��б���ʳ��ˮ�ͷ�̪����ֽ�ϣ���������ͼ��ʾ��װ�á������жϺ������ǣ� ��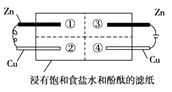
A.���ͭƬ��������ð��
B.�ұ�пƬ�ϵķ�ӦΪ2Cl-- 2e-=Cl2��
C.���ȹ۲쵽��ɫ�������Ǣ�
D.��ͭƬ�������������仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ֻ�������Ӷ�û�������ӵľ����ǣ� ��
A. �������� B. ԭ�Ӿ��� C. ���Ӿ��� D. ���Ӿ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ�����Ķ���������Ԫ��R��X��Y��Z��M��ԭ������������������ռ���������ڡ�Y��Mλ��ͬ���壬Yԭ�������������ǵ��Ӳ�����3����������Ԫ�����һ�����ӻ�����Q��ȡһ����Q��������ˮ�õ���Һ������Һ�еμ�ϡ����������Һ�������ij���������������Һ����Ĺ�ϵ��ͼ��ʾ������˵������ȷ����
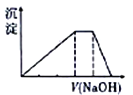
A. ��̬�⻯��ķе���Y>X
B. R���Z���������3��Ԫ�ؾ����γɹ��ۻ�����
C. �����ӵİ뾶��M>Y>Z
D. Y������4��Ԫ�ؾ����γ��������ֶ�Ԫ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ڵ�5������Ԫ��A��B��C��D��E��ԭ��������������A��B��C����Ԫ��ԭ�ӵ��Ӳ���֮����5��Bԭ�ӵ�����������������Ӳ�����2����D��һ�ֵ�����һ���������ζ�ĵ���ɫ���壬������ɱ��������E��A��ͬһ���塣
��ش�����������
��1��A��E���γɻ�����ף�����ˮ��Ӧ������H2��д���ĵ���ʽ��__________________��
��2������AlCl3��Ӧ�õ�NaAlH4������NaAlH4������Ҫ�Ļ�ԭ����д��NaAlH4��ˮ������Ӧ�Ļ�ѧ����ʽ��____________________________________��
��3��ijͬѧ��Ϊ���ö�������Ͼ���Ӧ��ϵ�еĿ��������������ᷴӦ������徭Ũ����������E�ĵ��ʷ�Ӧ���õ��������ʼ�Ϊ�����ļ���ȡ�ù���������ˮ��Ӧ�����ܲ���H2������֤���õ��ļ�һ���Ǵ����ġ��жϸ�ͬѧ������Ʊ����鴿�����ĺ����Բ�˵��������____________________________________��
��4��CԪ�ص���̬�⻯��Ҳ����һ���Ļ�ԭ����д������CuO��Ӧ�Ļ�ѧ����ʽ��____________________________________��
��5��B��D��E��ɵ�һ��������B����������Ϊ17.91%����ˮ��Һ��ʹ���Ը��������Һ��ɫ��д�����εĻ�ѧʽ��_______________�������£����ε�ˮ��Һ������Ũ���ɴ�С��˳��Ϊ��____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij������M�����ֳ�����Ԫ�����,Ϊ�˲ⶨ����ɽ�������ʵ�飺
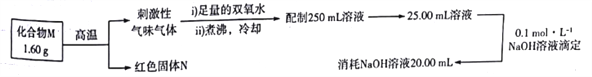
M�Ļ�ѧʽ����Ϊ�� ��
A. Cu2S B. CuS C. FeS2 D. Fe3S4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���й�ҩƷ������ȷ���ǣ� ��
A.ﮱ�����ú����
B.Һ�屣���ڴ���Ƥ������ɫϸ�ڲ���ƿ��
C.���ױ�����ˮ��
D.�ⵥ�ʱ�������ɫ���ƿ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com